A Proven Non-drug Treatment
Many Benefits of NeuroStar TMS Therapy
- FDA-Cleared
- Non-drug
- Non-invasive
- No common side effects of medications
- Not ECT
- Long Lasting Relief
- Covered by Insurance
- Proven to work

How it Works
Targeted Pulses
Magnetic energy similar to what is used in an (MRI) is used to stimulate the areas of the brain that are sluggish and causing depressed mood.
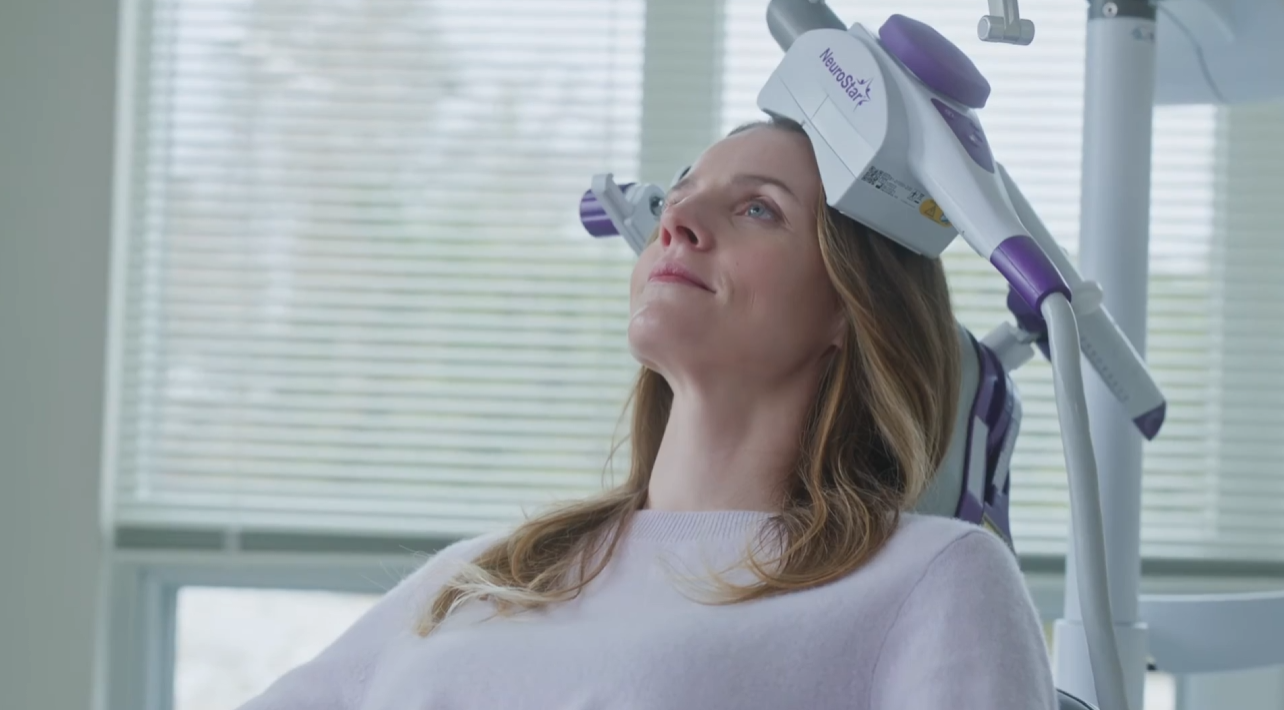
NeuroStar TMS is Safe and Effective
FDA-Cleared Treatment
Clinical trials have demonstrated the safety and effectiveness of NeuroStar® TMS Therapy in treating patients who have not benefited from prior antidepressant medication. NeuroStar TMS was studied in adult patients suffering from Major Depressive Disorder, all of whom had not received satisfactory improvement with previous treatments.
TMS for Teenagers
Safe and Effective for Adolescents
Nearly one in five adolescents in the United States experience a major depressive episode each year, and many look for treatment that feels manageable and consistent. TMS offers a structured, session based approach that helps maintain progress over time.
NeuroStar TMS has treated nearly 10,000 adolescents through more than 300,000 sessions. (Based on 169,000 global patients treated. Data on File, 2024)
improvement in depression symptoms
symptom relief (remission)
Kessler, RC et al. (2005). Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 62(6):593-602.
Frequently Asked Questions
TMS stands for transcranial magnetic stimulation. It is used to treat depression by stimulating the brain non-invasively using electromagnetic fields, similar to those produced by an MRI machine. During TMS Therapy, a magnetic field is administered in very short pulses to the part of the brain that research has demonstrated to be associated with depression. The typical initial course of treatment is about 19-37 minutes daily over 7 weeks.
In a study of “real-world” outcomes (meaning actual NeuroStar patients), 83% of people who completed the full NeuroStar treatment cycle experienced a measurable decrease in the severity of their depression, and 62% of those completing treatment saw full remission – meaning their depression effectively “went away.” [1]
The NeuroStar Advanced Therapy system uses short pulses of magnetic fields to stimulate the area of the brain that is thought to function abnormally in patients with depression. The magnetic field produces an electric current in the brain that stimulates the brain cells (neurons). This results in changes that are thought to be beneficial in the treatment of depression.
It usually takes time for healthcare insurers to establish coverage policies for newly approved treatments such as NeuroStar Advanced Therapy TMS. However, many commercial and Medicare plans have recognized the effectiveness of treating depression with TMS Therapy and now cover TMS as part of their plans. See here for a full list of insurance plans that cover TMS.
NeuroStar® TMS Therapy is non-systemic (does not circulate in the blood throughout the body), so it does not have side effects such as weight gain, sexual dysfunction, nausea, dry mouth, sedation, etc. The most common side effects reported during clinical trials were headache and scalp discomfort – generally mild to moderate – occurring less frequently after the first week of treatment.
No. NeuroStar TMS Therapy involves a unique method of using pulsed magnetic fields for therapeutic benefit. The intensity of the magnetic field is similar to that of the magnetic fields used in magnetic resonance imaging, or MRI. These techniques differ radically from the popular use of low intensity, static magnetic fields. These products deliver weak and undirected static fields that are not capable of activating brain cells.
[1] Sackeim HA, et al. (2020) J. Affect. Disord. 277:65-74.
Based on a real-world, retrospective study using CGI-S and a sample size of 615 patients.
© 2022 Neuronetics, Inc., Malvern, PA | 53-51670-000 Rev A
• Covered by most insurances
• FDA-cleared
